Overview
Welcome to the Society for Cardiovascular Pathology (SCVP) and Association for the European Cardiovascular Pathology (AECVP) web tutorial on interpreting antibody-mediated rejection by endomyocardial biopsy for cardiac transplant patients. The goal of this tutorial is to educate pathologists on a method of interpretation and on the findings one sees for this entity.
This tutorial contains 5 sections covering all aspects of antibody-mediated rejection. The menu on the left hand side is clickable and can allow the user to move directly to an area of interest. All images can be enlarged by clicking on them (click on the picture to shrink it down). A self-testing quiz using virtual slides of actual cases has been created to make sure you are able to determine cellular rejection and not confuse other histologic findings.
This tutorial has gone through a two-step process of document creation and editing. The members of this committee can be found on the about page and they are solely responsible for the material contained herein. A complementary tutorial focusing on cellular rejection can be found here.
History and evolution of the ISHLT working formulation for AMR
Cardiac AMR has evolved over 25+ years from a controversial consideration to an established entity. In 1989, Hammond et al reported a pattern of “acute vascular (humoral)” rejection in 6 patients in Utah. The 1990 ISHLT Working Formulation (ISHLT WF) briefly addressed AMR but further study was needed. Reports from other centers followed, and in 2005 and 2006 the revised ISHLT WF AMR criteria were formally defined. The Banff 2009 conference highlighted a lack of uniformity in applying these criteria and variability in staining techniques for C4d. In 2010, a multidisciplinary effort by the ISHLT further refined the clinical and pathologic definitions of AMR. This work culminated in the current (2013) pathologic ISHLT WF.
Clinical and pathologic spectrum of AMR
Manifestations of AMR in tissue, serum, and patients each exist in a spectrum, and varying definitions have made it very difficult to compare “apples to apples” in the AMR literature. Much of the historic controversy around AMR has to do with semantics, terminology, and varying clinical and pathologic definitions. The 2013 ISHLT does not resolve all of these, but does standardize the diagnostic elements, with the aim of facilitating multicenter studies based on uniform criteria.
AMR is also dynamic, resulting from the complex interplay of several factors that each evolve over time. These include the magnitude (titer) and potency (isotype) of the inciting antibody, the efficiency of that antibody at fixing complement, and the influence of complement regulators in the graft. Because of this, several different “forms” of AMR are described, including:
Hyperacute rejection: Perhaps the first and best recognized form of AMR (or vascular/humoral rejection in the early literature). It is the most extreme end of the AMR spectrum and is the result of high titer pre-formed antibody immediately affecting an allograft after implantation. Because of the abundance and effectiveness of the pre-formed antibody, complement is activated immediately and severe damage to the endothelium ensues. Complement split product chemoattractants cause an influx of neutrophils and other leukocytes. The coagulation cascade is also initiated. The implanted grafts can turn dark and stop functioning before the surgeon’s eyes. Histologically, hyperacute rejection is characterized by diffuse hemorrhage, transmural vascular inflammation and thrombosis, and abundant neutrophils. Thankfully, hyperacute rejection is vanishingly rare today thanks to more precise tissue typing methodologies and better graft-recipient matching practices.
Acute AMR: generally refers to a clinically significant (with graft dysfunction) episode of AMR either occurring shortly after transplantation or else as an abrupt change after a period of stable graft function. It is generally assumed (though not yet data supported) that these episodes are associated with high titers of anti-donor antibodies and that immunopathologic and morphologic features of AMR are more often present with this form. When all the features are present, this is the type of AMR for which there is greatest consensus and agreement.
Subclinical AMR: When immunopathologic and/or histopathologic, and/or donor specific antibody studies indicate AMR, but there is no graft dysfunction by clinical or imaging studies. This has also been referred to as silent, latent, or asymptomatic AMR. This situation is the source of some controversy and the implications for short and long term prognosis (and therefore the most appropriate treatment approach) are not clear. Many assume that the statistical association between AMR (defined variably in different studies) and Cardiac Allograft Vasculopathy (CAV) is explained by ongoing endothelial injury in subclinical AMR. Conclusive data supporting this are lacking.
Late AMR/Recurrent AMR: This refers to an AMR episode (with or without graft dysfunction) occurring either for the first time or as a recurrent episode at some significant duration after transplantation (often a year or more) or after a significant interval since the last AMR episode. Though not data supported, there is an assumption that this form of AMR results from de novo or latent memory type immune recognition of the graft rather than pre-formed antibody or presensitization. There is mounting evidence that AMR episodes occurring after the first year post-transplant almost always occur in patients who had some evidence of AMR shortly after transplant (or would have if studies had been done). Retrospectively staining an early biopsy for C4d may help clarify this if the question ever arises in a given patient.
Chronic AMR: This is a more nebulous term that has been used to define several different conditions. In some reports it is used synonymously with CAV, without respect to ISHLT WF features of AMR. Others have used the term to describe the scenario of late or recurrent AMR (as above) – equating acute with shortly after transplant and chronic with longer after transplant. This term has also been used to refer to morphologic findings seen at autopsy or explant (and less reliably in biopsies), often associated with CAV, in which there is increased fibrosis, reduced capillary density, and ultrastructural changes in capillary basement membranes. Complement staining is often negative when these changes are seen, but these patients frequently have had positive staining in the past. Clinically, there may be ventricular stiffening (diastolic failure) and a high incidence of sudden arrhythmic cardiac death. This entity is not well understood and not widely agreed upon.
Incorporating pathologic AMR diagnosis into the clinical picture
The 2013 ISHLT WF defines biopsy-based pathologic AMR , but this is only one factor in the clinical diagnosis and treatment of AMR. The presence of DSA, imaging and hemodynamic assessments of graft function, and other signs and symptoms of graft failure must all be considered by the clinical team. This integration is fundamental for patient management but challenging and remains open to debate between centers. One of the primary controversies revolves around treating patients with “asymptomatic/subclinical” AMR. In these patients with normally functioning grafts, the risk of intensifying immune suppression (further predisposing to infections and neoplasms) must be weighed against the potential benefit of preventing later cardiac allograft vasculopathy (CAV) and other complications.
An online survey of 184 ISHLT members showed greatest agreement for treating AMR with graft dysfunction regardless of pathologic AMR severity. Most centers also would treat AMR if DSA was present, with or without graft dysfunction and with any degree of pathologic AMR severity. In the absence of both graft dysfunction and DSA, pAMR1 would be treated by 30% of respondents, pAMR2 by 50% of respondents, and pAMR3 by 70% of respondents, so pathologic AMR (link for pAMR definitions below) is not entirely ignored.
Approaches to AMR surveillance
Unlike cellular rejection, AMR requires multiple inputs (histology, immunopathology, serum donor specific antibody (DSA) testing, and clinical measures) for diagnosis and treatment. These additional tests add expense and complexity, so their use in coordinated AMR surveillance strategies has varied from center to center. AMR surveillance strategy planning must account for the fact that individual morphologic, immunopathologic, serologic, and clinical features of AMR fluctuate over time and may not always coincide. The 2013 ISHLT WF recommends assessing all biopsy for AMR by histopathology, and outlines 3 indications for adding biopsy immunopathology:

The ISHLT WF also recommends an AMR surveillance schedule for biopsies in the 1st year after transplant:
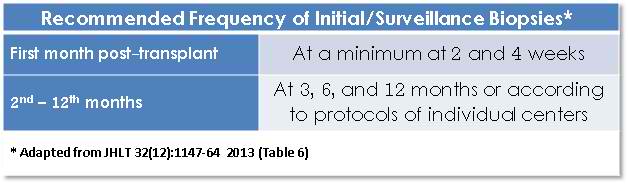
The ISHLT WF also cautions about immunopathology at < 2 weeks since perioperative ischemic injury and other changes may cause false positive results. It also recommends more frequent testing for any patients with positive immunopathology; continuing on all biopsies until negative results are seen. Biopsies should ideally be spaced 2 weeks apart, given the clearance kinetics of complement staining. Since AMR may occur after the 1st year as well, many centers continue AMR surveillance into subsequent years, but this is not addressed in the 2013 ISHLT WF.
Working Formulation
The 2013 ISHLT-WF addresses the pathologic diagnosis, grading criteria and reporting of cardiac AMR. It reflects a collaborative effort by pathologists from institutions in the US and Europe, acting as a subgroup of the ISHLT Pathology Council. Part of their effort included an interobserver reproducibility study focused on specific histologic findings using digitized slides from collected AMR cases. Results are mentioned in the 2013 ISHLT WF report; they showed “good”, but far from perfect scoring agreement.
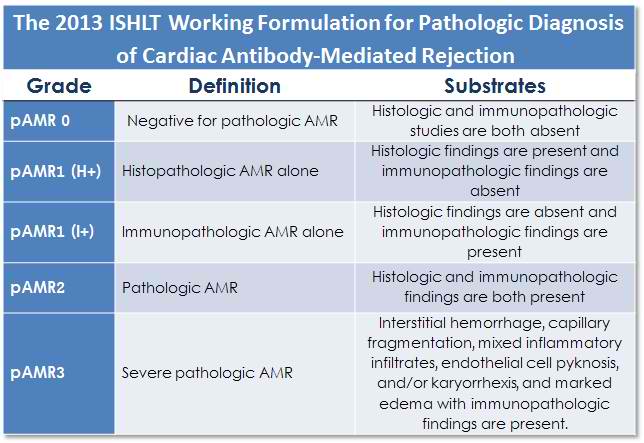
The 2013 ISHLT-WF differs from previous iterations by addressing AMR as a pathologic entity, separate from clinical parameters such as graft dysfunction and donor-specific antibody. Pathologic AMR, designated “pAMR”, is graded on the basis of a combination of histopathological and immunopathological findings as follows:
pAMR0 – Negative histologic and immunopathologic evaluation (similar to ISHLT 0R for cellular rejection.
pAMR1 – Characterized by either histopathological changes [pAMR1 (H+)] or immunopathological findings [pAMR1 (I+)] but not both. Must designate either:
pAMR1(H+) – “Activated” endothelial and intravascular mononuclear cells by routine histology (H&E) with negative immunopathology studies.
pAMR1(I+) – Positive immunohistochemical or immunofluorescence staining according to criteria outlined further into the tutorial, with normal biopsy histology.
pAMR2 – Characterized by both histopathological changes and immunopathological findings (i.e. pAMR (H+) + pAMR (I+) = pAMR2).
pAMR3 – Severe antibody mediated rejection. This pattern is very rare, especially in the current era. It is similar to early descriptions of hyperacute rejection and shows significant overlap with severe cellular rejection – ISHLT 3R. This pattern is characterized by widespread myocyte injury, hemorrhage, prominent neutrophils, capillary fragmentation (pyknosis, karryorhexis), and marked edema. Complement staining is required for this diagnosis. Fibrin staining (by immunofluorescence) is reported to be a feature of severe AMR. These cases are usually associated with profound hemodynamic dysfunction and poor clinical outcomes.
Mixed rejection (AMR + ACR)
The concept of coexisting cellular and antibody-mediated rejection is not new. In some of their earlier published work Hammond and colleagues as well as others have noted the mixed patterns. Currently, most transplant pathologists recognize that biopsies with the histopathologic and/or immunophenotypic findings of AMR also have a component of ACR. In most cases the ACR is low grade (Grade 1R in the 2005 revised grading scheme or focal mild/Grade 1A or diffuse mild/Grade 1B in the original 1990 scheme). Less commonly, however, higher grade of ACR such as 2R or 3R are encountered in association with AMR.
The ability to recognize mixed rejection obviously depends on the surveillance strategy for AMR. The 2013 ISHLT WF recommends that each component be separately evaluated and graded according to established criteria.
The concept of separation between the cellular and antibody mediated arms of the immune system may not be as rigid as theoretically proposed. There is increasing interest the commonality of the pathways.
Histopathology
Low Power
Cellularity
At scanning power (x20-40) the feature suspicious of AMR is a “busy” look to the myocardium – a diffuse “B” pattern of increased cellularity giving a “busy B” appearance that, unlike cellular rejection, is present throughout the biopsy.
Interstitium
The “busy” appearance may be accompanied by interstitial edema, with separation of myocytes. In severe cases there may be extravasated red blood cells/hemorrhage as well, although this is very unreliable in biopsy tissue given the traumatic bioptome sampling. Severe AMR also typically shows widespread myocyte injury, such that there is overlap between severe AMR and grade 3R rejection.
Vasculature (capillaries vs. venules/arterioles)
The impression that the increased “cellularity” is in the vicinity of the microvasculature can sometimes be appreciated at a slightly higher power (x100). Cells within arterioles and larger venules should generally be ignored.
High Power
Endothelial Cells
Higher magnification (x200-400) allows inspection of capillaries and venules in the myocardial interstitium. Interstitial capillary injury is characterized by large and prominent (“swollen”) endothelial cells. Enlarged nuclei and/or and expanded cytoplasms appear to narrow or occlude the lumens. Similar endothelial cell changes can also be seen in the setting of ischemic injury or settings besides AMR, so this finding should be interpreted in context (i.e. other ischemic injury features, biopsy site, presence of intravascular macrophages, etc.). Endothelial cells with pyknosis and/or karyorrhexis can reflect severe AMR.
Intravascular mononuclear cells
At high power, intravascular macrophages often distend and fill the capillary and venular lumens. These can be difficult to distinguish from endothelial cells in some cases, but if they are completely detached from the vessel wall and show typical morphologic features of macrophages, one can be reasonably certain of their identity. CD68 immunohistochemistry is confirmatory.
The prevalence of macrophages is variable (quantitative criteria discussed under CD68 below). The “swollen” endothelial cells are usually seen more diffusely. Most cases of AMR display diffuse or multifocal patterns of “activated mononuclear cells”. If CD68 staining is not routinely done, cases with even focally prominent intravascular mononuclear cells should prompt staining for CD68. In some subtle cases the number of CD68 positive macrophages will be surprising.
Edema
Edema may be appreciated at lower power, but on closer inspection should show pale blue proteinaceous material in the interstitial space. The finding of edema may vary from sparse accumulations to prominent widespread collections, especially in cases of severe AMR.
Edema should not be confused with artifacts due to biopsy or histology preparation-induced spaces (which lack the pale blue staining). Edema should be also distinguished from interstitial fibrosis. The wavy fibrils of collagen or a connective tissue stain, such as Masson trichrome, can be used to confirm interstitial fibrosis.
Edema is also not specific for AMR. It can be seen in the setting of ischemic and perioperative “harvesting” injury.
Inflammation
Inflammatory cells other than macrophages may also be prominent in AMR. These are often present in the interstitium as well as intravascularly. This raises the issue of “mixed” rejection (discussed below), but in the setting of pure AMR, the inflammatory cells could include neutrophils and eosinophils (which are rarely seen in cellular rejection) as well as lymphocytes (especially T and NK cells). Interstitial inflammation is more prominent in severe AMR, in combination with edema, hemorrhage, endothelial cell pyknosis/karyorrhexis and extensive myocyte damage.
Immunopathology
Approaches to Immunopathology
Immunopathologic studies can be performed on paraffin or frozen tissue, with comparable sensitivity and specificity, although frozen immunofluorescence is generally considered to be the gold standard. The ISHLT WF allows laboratories to use whatever method they prefer based on their capabilities and individual preferences, but it is critical to have appropriate staining controls.
There are important logistic considerations with immunofluorescence, since the biopsy tissue must not be placed in formalin. Since the frozen tissue is separated from the other pieces examined by routine histology, reviewing the frozen section H&E from this piece should not be overlooked. The specific antibody staining panels differ for each method and the ISHLT WF has outlined required and optional sets of stains for each (see IHC Table and IF Table).
Immunopathologic diagnosis
Immunohistochemistry (IHC) for paraffin-embedded tissue and immunofluorescence (IF) for frozen-tissue will be treated separately as they entail different antibody panels and a different mode of interpretation.
Low and High Power Examination
Like routine histology, an algorithmic approach to immunostain interpretation is helpful. An approach based on scanning/low power (x20-40) followed by high power (x200-400) is suggested.
The first low power biopsy assessment helps:
- Ensure adequate myocardial tissue and section quality
- Assess overall pattern of staining (focal, multifocal, diffuse)
- Identify areas to evaluate on higher magnification and areas of artifactual staining to avoid
High power examination helps:
- Assess intensity, pattern (granular versus homogeneous/linear), and completeness of circumferential capillary staining for complements
- Localize CD68+ macrophages (inside or outside the capillary walls)
Paraffin Immunohistochemistry
Overview
Frozen tissue IF as an immunopathologic tool in evaluating AMR has been in use longer than paraffin IHC, but with modern antigen retrieval methods, IHC methods are essentially equivalent and now more commonly used in routine practice. All staining protocols must be optimized and validated appropriately and every staining run should include appropriate control tissue sections. Biopsy adequacy is also important, and the ISHLT WF recommends that 3 pieces of myocardium should be available for paraffin IHC.
Recommended vs. Optional Stains
Surveys have demonstrated wide variation in practice, highlighting the need to define appropriate antibody panel guidelines for the IHC immunopathology. The ISHLT WF proposes primary and secondary antibody panels for IHC.
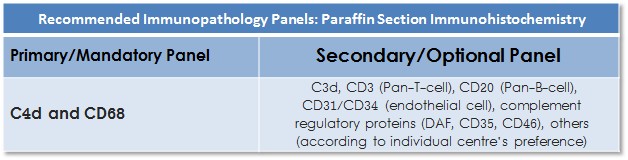
Primary/Mandatory Panel
C4d
Capillary deposition of complement degradation product C4d is a key immunopathologic AMR finding. The binding of C4d to endothelial surface proteins is covalent and therefore persists much longer than other indicators of complement activation (like C1q).
Available commercial C4d antibodies for paraffin IHC are limited and almost exclusively polyclonal reagents. The following table summarizes results of a 2009 European survey of C4d staining by IHC:
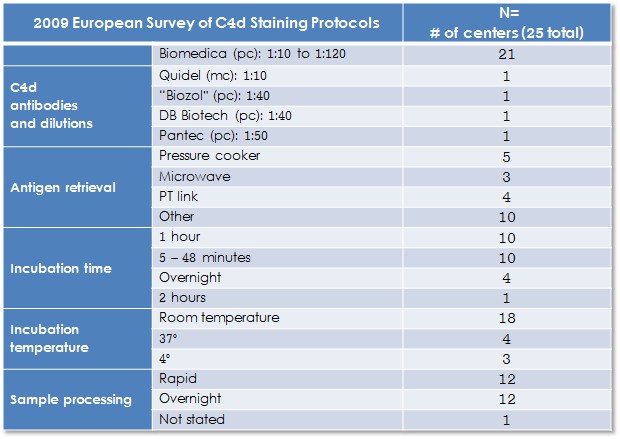
There are 3 essential elements when evaluating C4d by IHC:
1. Specificity: Only interstitial capillaries in intact myocardium should be assessed. The staining of arterial, arteriolar and venular staining should be ignored, except as possible internal controls with some antibody clones. Staining of these structures should be linear (or finely granular) and encompass the entire circumference of the vessel. The terms “donut” or “elliptical” have been used when the capillaries are viewed in cross-section or tangential/longitudinal cuts, respectively.
2. Intensity: Due to either assay performance or the temporal evolution of AMR the intensity of staining may vary. Occasionally, variation may be seen from region to region in the same biopsy. The 2013 ISHLT WF defines a 3-degree scale for evaluating intensity.
3. Distribution: C4d capillary staining is not always uniformly and diffusely distributed. The 2013 ISHLT WF proposes a scoring system for C4d distribution as well.
The interpretation (positive or negative) of C4d capillary depends on distribution and intensity.

CD68
Although more controversial, CD68 has been included in the primary/mandatory panel because:
- Some cases with the same AMR histopathologic features may be C4d(-) but CD68(+).
- Intravascular CD68+ macrophages seem to independently predict C4d deposition in capillaries and circulating DSAs.
- In some cases, accumulating intravascular macrophages may not be apparent on routine histology, but are clearly demonstrated by CD68 staining.
By including CD68 in the criteria for immunopathology, there is potential for “double counting” a single attribute of AMR (accumulation of intravascular monocytes). A case with this finding on routine histology that is confirmed by CD68 staining would be considered to meet both histologic and immunopathologic criteria for AMR (so pAMR2), even though complement staining may be negative.
The essential elements in evaluating CD68 immunostaining results are:
1. Positive cells should be restricted to the luminal spaces of capillaries and small venules. The pattern is described in the 2013 ISHLT-WF as “chain-like, linear, or beading staining profiles within interstitial capillaries or small venules aligned in longitudinal orientation and clusters or beading within the vascular lumen in cross-section”. It is not always possible to distinguish intravascular from extravascular macrophages.
2. The staining intensity of CD68+ is generally uniform, but the distribution is variable. Interpretation is further complicated by the fact that macrophages are a normal resident cell in biopsies. The 2013-WF Working Group suggests that the spectrum of “normal” includes prominent macrophages in up to 10% of the biopsy area. Beyond this, the distribution should be scored as focal (score 1): 10%-50% and multifocal/diffuse (score 2): > 50%.

CD68 (and C4d) should be assessed on intact myocardium away from areas of well recognized ACR, Quilty lesions, biopsy site scars and areas of ischemic-related damage or necrosis. Interstitial macrophages can also be found in cellular rejection, ischemic injury, infection and healing biopsy sites. So it is important to ensure the CD68 positive cells are confined to intravascular spaces when assessing for AMR.
There are more than one commercially available antibody clones for CD68. The KP1 clone is most commonly used in the literature, but the PGM1 clone is also acceptable. Also, CD68 has broader leukocyte specificity than some newer macrophage/monocyte lineage markers such as CD163. Studies using CD163 as an immunopathologic assessment of AMR have not yet been conducted.
Secondary/Optional Panel
C3d
Since C3 cleavage occurs further down the complement cascade than C4 activation, it is felt by some to be a more reliable indicator of complement cascade completion (i.e. without interruption by complement regulators). Until recently, the commercially available antibody clones for C3d could only be used in frozen section immunofluorescence. Several labs are successfully using clones in paraffin immunohistochemistry, but often report high background staining. Because experience with this is limited, C3d is not included in the primary panel for paraffin section IHC, even though it is on the primary frozen immunofluorescence panel.
The 2013 ISHLT-WF recommends that criteria for assessing intensity and distribution of C3d staining should be the same as those for C4d evaluation. C3d findings should not be considered in the immunopathologic interpretation of AMR (since it is an optional marker).
CD31/CD34
These antibodies are very useful in assessing integrity and density of the microvascular network and provide a basis for comparison for a detailed evaluation of C4d and C3d percentage distribution, especially when digital image analysis algorithms or double staining strategies are used.
Both antibodies have well established protocols since they are widely used in surgical pathology. They are essentially interchangeable for endothelial staining although CD34 may also react with some leukocytes and other cells as well.
CD3
This pan T-Cell antigen has been included in the secondary or optional panel since it is used by some as an attempt to better characterize the inflammatory cell component, especially the mononuclear cells within capillaries. There is morphologic overlap between some patterns of cellular rejection and the histologic features of AMR. CD3 may also be helpful in establishing the diagnosis of “mixed” rejection (concomitant acute cellular rejection and AMR). It should be noted that the ISHLT-WF experts agreed that it was not necessary to use CD3 staining routinely for the diagnosis of acute cellular rejection.
CD20 & CD138
Antibodies directed against the pan B-Cell (CD20) and plasma cell specific (CD138) antigens have also been included in the secondary IHC panel since they are used by some to better characterize inflammatory cell constituents. In the setting of a significant number of positive CD20 and/or CD138 cells, the possibility of lymphoproliferative disorder should be addressed (and additional studies such as EBV considered). Some pathologists also use CD20 to help differentiate Quilty effect from rejection.
C5b9
This antibody has been used as a surrogate for the detection of membrane attack complexes on capillary endothelium. It is applied for monitoring of therapeutic usage of specific humanized monoclonal antibodies (such as eculizumab). With these therapies there may be initiation of the complement cascade (so positive staining for C4d and C3d), but blockade of the cleavage of C5 step prevents it from running to completion (so no formation of membrane attack complexes). Only a few centers have experience with immunohistochemical staining of paraffin sections and since use of eculizumab and similar therapies is quite limited, this stain is considered optional.
Frozen Section Immunofluorescence
Recommended
Frozen tissue IF has been a longstanding immunopathologic tool to evaluate AMR. The ISHLT-WF also includes recommended and optional panels for IF stains.

C4d and C3d
Other than the differing techniques and microscopy methods, the interpretation of C4d and C3d stains is identical to IHC techniques. Specifically, capillary staining should be identified being mindful of the percent of tissue involved and staining intensity.
Optional Stains
Other complement components (C1q/C3c)
Before C4d and C3d antibody clones were commercially available (C4d was not widely used before 1995), many laboratories were using other complement products that are commonly employed in kidney and skin biopsy immunofluorescence. Results were variable, but in general staining for C1q and C3c are considered to be too transient to be reliably detected for very long after complement activation. C1q and C3 are not felt to have a role in routine diagnosis of AMR.
A recent modification of flow cytometry based donor specific antibody (DSA) tests uses reagent complement to “spike” samples in order to determine if a detected anti-donor antibody is able to fix complement or not (by subsequently detecting C1q by flow cytometry). This in vitro procedure is very different from what occurs in vivo in terms of C1q metabolism. C1q binding is transient in vivo and staining biopsies for C1q cannot reliably provide the same data regarding complement fixation as the C1q DSA assays.
Ig Heavy Chains
Staining for immunoglobulin heavy chains (IgG, IgM, IgA) was also performed by some laboratories before complement antibodies were widely available. Staining for heavy chains may be seen in biopsies early after transplant in more severe cases and depending on the timing of the biopsy in relation to the oscillation of DSA titers, staining for heavy chains may also be seen later on. Staining for heavy chains detects antibody still bound to endothelium, a phenomenon that is also recognized to be more transient than the covalent binding of C4d. Because of this limitation, routinely staining for IgG, IgM, and IgA is not recommended in routine AMR surveillance by immunopathology.
Fibrin
In more severe rejection episodes the degree of endothelial damage may be so significant that coagulation cascade initiation also occurs. This rarely results in overt occlusive thrombosis of vessels, but more subtle deposition of fibrin in capillaries and leaking out of damaged capillaries into the surrounding interstitium has been described in the literature. When this pattern is seen, it indicates a more severe episode of AMR and patients whose biopsies have shown positive fibrin staining have a worse prognosis in small single center studies. The additive prognostic value of fibrin over simply recognizing higher grades of rejection in the ISHLT-WF has not been rigorously studied. This IF stain is not widely used.
HLA-DR
This stain is similar to CD31/CD34 by paraffin immunohistochemistry and has similar utility. The more important significance of HLA-DR staining, however, is reported to be its ability to highlight subtle damage to capillaries and has been touted as a means of assessing capillary integrity. While quiescent capillaries show crisp linear staining of endothelium, injured capillaries show more of a “frayed” or “feathered” pattern of staining with stellate projections from the capillaries into the surrounding interstitium. This pattern is thought to be helpful in identifying more severe episodes of AMR as well as “chronic AMR”.
Other (regulators of complement)
Activation/initiation of the complement system does not always result in its completion with membrane attach complex formation. There are several checkpoints and extrinsic regulators of the catalytic events that can prevent this. Some of these are thought to play a role in the development of immunologic tolerance. Some laboratories have developed immunofluorescence staining protocols for some of these factors such as CD59 and CD55/decay accelerating factor (DAF). The commercially available antibodies for these are limited and need further development (most require overnight incubations) before they can practically be used in routine diagnosis. The staining patterns for these are described as granular and limited to capillaries. Interpretation is more difficult than for C3d and C4d.
Artifacts
Immunohistochemistry
Serum staining
Non-specific staining of retained serum with vessels is commonly seen, but on close inspection it can easily be distinguished from bound C4d on the endothelium. “True” positive C4d staining will show crisper, more linear and circumferential staining. “False” serum staining may also show “bubbles” or curved edges projecting into the lumen.
Arteries
Arteries have an internal elastic lamina that often stains with C4d. On one hand, this can be seen as a helpful internal positive control, but should not be taken as evidence of AMR.
Connective tissue / elastic
Any staining of the interstitium should be ignored, although in severe AMR this may represent damage to the microvasculature and leakage of plasma proteins. Elastin fibers may be prominent beneath the endocardium or in areas of scarring and may fracture during microtomy and spread across the tissue sections. These fibers often stain strongly with C4d.
Sarcolemma
Occasionally, there is membranous staining of myocytes. This is not considered to be evidence for AMR and should be ignored.
Ischemia
Ischemic myocytes will generally show strong staining for C4d throughout the entire cell. This can also be seen in non-transplanted hearts as well. Correlation with the H&E stained sections is helpful in confirming foci of early ischemic injury. This is most often seen in the first weeks after transplant or later on in patients with CAV. CD68 staining may also be seen in areas of recent ischemic injury, but macrophages are localized to the interstitium rather than intravascularly.
Quilty effect
Endocardial lymphocyte aggregates or Quilty lesions are discussed in the ACR tutorial. C4d staining of Quilty lesions is largely limited to the endocardium where it is often linear and may extend beyond the margins of inflammation. Capillary C4d deposition has been associated with Quilty effect, but is usually focal, so not considered significant in the ISHLT WF.
Immunofluorescence
Autofluorescence
The main potentially confounding artifact in frozen tissue immunofluorescence is autofluorescence, or the broad spectrum emission spectra of naturally occurring cellular materials. H&E stained frozen sections should be performed to appreciate the relative amounts of myocardium, endocardium and connective tissues sampled prior to assessing immunofluorescent stains. Positive and negative control tissue stains can also be helpful in assessing this.
Lipofuscin
Lipofuscin pigment is often present in cardiac myocytes, appearing and as brown granules in the sarcoplasm often with perinuclear clustering by light microscopy. By fluorescence microscopy the granules demonstrate strong autofluorescence. While primarily intracellular, lipofuscin may migrate during the frozen section processing and may also be taken up by macrophages. Fluorescence microscopes equipped with filter and cube combinations that allow dual green/red emission visualization can be helpful in cases with abundant lipofuscin. The antibody label will appear green and the lipofuscin red.
Elastin
Elastin and some collagens also autofluorescence. Elastin autofluorescence is most prominent in larger vessels but internal elastic lamina elastin may be evident even in smaller vessels and produce distracting foci of fluorescence. Understanding that only interstitial capillary C4d deposition is diagnostic of AMR and familiarity of larger vessel autofluorescence on negative control slides should help avoid distraction by these foci.
Sarcolemma
Perimyocytic C4d and C3d staining giving the appearance of sarcolemmal staining can also be seen and some have suggested this is more common in the setting of resolving AMR. Such staining may persist for weeks after clinical resolution of the AMR episode. Staining is diffuse and not limited to the true positive “donut” pattern of complement deposition noted in acute AMR.
Drying artifact may produce this pattern of sarcolemmal staining. Negative controls are again helpful in sorting this out.
Pediatric Biopsy Considerations
Diagnosis of AMR in the pediatric population
Published prevalence of AMR after pediatric heart transplantation is higher than that reported in the majority of adult centers and ranges from 10% to 59%, depending on whether cardiac dysfunction is required for AMR diagnosis. Like in the adult population, subclinical AMR frequently occurs in the pediatric population. The higher incidence is likely due to a combination of sensitization and medication non-compliance, particularly in adolescents.
The pathological diagnosis of AMR in the pediatric population is no different than adults, relying on the same combined histology and immunohistochemistry approach used in the adult population. The ISHLT WF for pathologic scoring of AMR severity is applicable to the pediatric population.
Biopsy Size & Cellularity
As already stated in the cellular rejection tutorial, endomyocardial biopsies performed in the pediatric population are characterized by smaller size and by an increased impression of cellularity. At low power, the latter should not be mistaken with a “busy B” pattern. At high power, careful assessment of the microcirculation is warranted to identify significant intravascular mononuclear cells. One should be aware that plump endothelial cells are frequently observed in the pediatric biopsies and should be interpreted with caution.
Sensitization in the pediatric population
According to the ISHLT registry, the percentage of sensitized patients, as defined by panel-reactive antibodies (PRA) of >10%, increased from 20% in 2005 to 29% in 2010. Sensitizing events in these patients include transfusion of blood products (especially platelets), use of ventricular assist devices, infections, and previous surgery for congenital heart disease, especially with the use of human homograft tissue. In the Pediatric Heart Transplant Study database and the ISHLT registry, congenital heart disease were associated with both increased risk factor for death after transplantation and of developing PRA of >50%. Having a Norwood procedure, prior listing, and sternotomy before listing were also all significant risks. Elevated PRA in children listed for heart transplantation is associated with longer waiting times to transplantation and increased pre-transplant mortality. High PRA is also associated with a 2-fold increase in early post-transplant mortality.
ABO incompatible allografts
Infants less than 1 year of age lack antibodies against blood group antigens since humoral immunity to carbohydrate moieties is poorly developed at this age. This allows for transplantation across blood types. Although AMR has been reported in pediatric ABO incompatible heart transplantation, infants who received these transplants had equivalent one-year survival. Immune tolerance to the graft ABO type develops (accommodation), though there is the theoretic possibility of bound anti-ABO antibody on the graft endothelium initiating the complement cascade leading to C4d positivity but with normal histopathology and no graft dysfunction.
Reporting Results
Pathology Reporting
ISHLT diagnostic terminology from the 2013 ISHLT WF is recommended. Additional comments, preferably in a “searchable” field in the electronic report so data can be retrieved, on C3d and CD68 staining if performed, and on the intensity and extent of C4d staining, can also help eventual understanding of the clinical import of the various pathologic features of AMR. A representative template for reporting is available here.
Communicating Results
Reporting by telephone or fax or secure email/texting of positive (and negative) results should speed clinical decision-making and treatment.

Future Directions / Areas of Research
Prognostic significance of C3d staining
As noted above, C3d staining by immunohistochemistry is not included in the 2013 ISHLT-WF. However, it is included in the recommended immunofluorescent panel. The experience at some centers, including Cleveland Clinic and Utah, has been that C3d immunohistochemical positivity identifies patients at increased risk for allograft dysfunction and death. Centers performing and reporting C3d staining have treatment algorithms incorporating this information. Certainly whenever C3d staining is performed it should be reported, if only to allow collection of data on the clinical import of C3d status.
Weak C4d staining
Additionally, inclusion of information on weak C4d staining or on incomplete C4d staining (fewer than 50% of capillaries are stained) is advisable in order to collect data to correlate with DSA and graft dysfunction. If weak or focal C4d staining is only very rarely accompanied by DSA or graft dysfunction it will provide additional rationale for considering this weak or focal staining to be ‘negative’ for pAMR.
pAMR1
In the ISHLT WF, pAMR2 is considered definite with all features present. pAMR1 is more controversial and the appropriate clinical response to pAMR1 depends mostly on graft function and DSA. Various centers’ responses to pAMR1(I+) and pAMR1(H+) have yet to be catalogued. The inclusion of both pAMR 1(H+) and pAMR1(I+) in the “Suspicious for AMR” category suggests they are pathologically and clinically equivalent; this has not yet been confirmed by systematic study and it should be the focus of additional study. It is possible that the release of various cytokines, associated with infection or other non-rejection inflammatory responses, will result in endothelial cell activation and prominence and even leaking of plasma into interstitial spaces as edema, without the binding of C4d. If true, this would suggest that pAMR1(I+) is more likely associated with DSA than pAMR1(H+). It will be interesting to see if the incidence of graft dysfunction differs in pAMR1(H+) and pAMR1(I+). It is also worth collecting data on pAMR1(I+) and pAMR2,3 indicating if the positive immunologic diagnosis is based on C4d, C3d, and/or CD68 positivity. Again, eventual correlation with DSA and graft function will be very useful in defining the appropriate clinical response to various manifestations of pAMR.
Correlating pAMR with DSA
Studies in both renal and cardiac allograft recipients have shown a positive correlation between C4d staining and circulating donor specific antibodies, making C4d perhaps the best single marker of AMR. However, it should be noted that recent studies in renal allograft recipients have demonstrated C4d negative antibody-mediated rejection in renal allografts (Haas review and 2014 Banff 2013 Meeting Report) so it should be noted that C4d is not an absolute marker of AMR.
Mixed rejection
Although at present there are not sufficient data in the literature, one of the goals for the future is to work on understanding and defining mixed rejection, the relationship between ACR and AMR and the pathways involved when coexisting.